Hi Rory,
I very well realize the importance of replicates as well as your advice in this forum multiple times to other questions earlier that no meaningful statistics can be done without replicates.
However, the data I have received to analyze has bad replicates. There are three matched pairs for two conditions and the biologist says that they can be loosely considered as replicates, but the initial correlation heatmaps indicate that they are quite far apart. As a result, when I use DiffBind, I don't see more than 1 differential binding site with default FDR. So, I set th=1 to get the full report.
However, my question is that if I wish to treat the three pairs separately, two at a time and just compare them without treating them as replicates, what can I do?
I see that the documentation for "dba.contrast" says the following:
minMembers when automatically generating contrasts, minimum number of unique samples in a group. Must be at least 2, as replicates are strongly advised. If you wish to do an analysis with no replicates, you can set the group1 and group2 parameters explicitly.
So, if I have just two samples, one per condition, is there a way to just get the output of fold changes? That is, process them using counts and then get the scores and outputs with fold changes? I can then potentially give these results to the biologist.
Any advice would be greatly appreciated.
Thanks a lot!

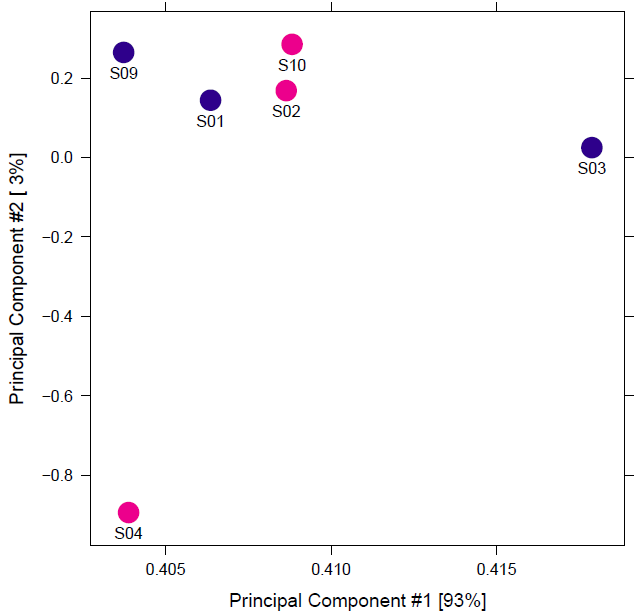

Better tell the biologist to repeat the experiment, unreplicated "results" are guesses rather then results, only creating ghosts to chase rather than a true hypothesis. ATAC-seq is (by experience) a very robust experiment, so if some replicats fail that means that probably the "surviving" replicates are also not of good quality, so the unreliability of unreplicated results is confounded even more by (probably) bad sample quality.
Thanks for the comment. Point taken, but, as you know, it is not so easy to tell them to simply repeat the experiement. Costs, time, etc. come inbetween. So, I can tell her that this is the best we can do by saying that it's not possible to get pValues without good replicates. However, like usually done in RNAseq DE, they expect me to at least give a list of fold changes for all the potentially differentially bound regions.
So, it would be great if I can get the steps to just compare two samples without considering them as replicates and get some fold changes.